HO2ME / Camera per ossigenoterapia topica
HO2ME normobaric chamber is made up of two transparent polycarbonate injection moulded half cases and a cover certified for healthcare applications. The pieces are interlocked by a simple “open and close” joint, without any gaskets.
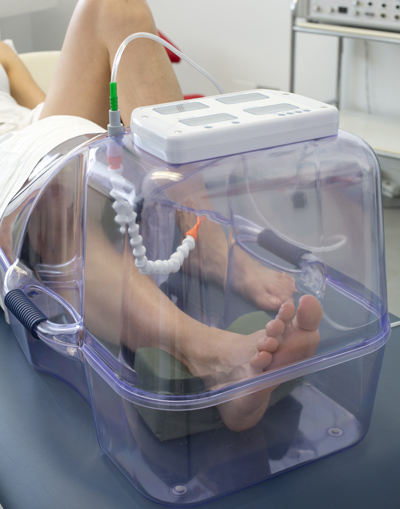
The proper oxygen flow from the HO2ME device to the limb of the patient is ensured through a POM articulated hose, while the normobaric Chamber is connected to the oxygen source through a special fast coupling external medical tube.
The oxygen source can be either a Medical Oxygen cylinder provided with a humidifier, if the treatment is performed at home, or the oxygen generating system of the Hospital. A safety valve prevents any overpressure and a suitable connection sleeve in canvas is applied to avoid any excess oxygen leakage from the normobaric Chamber. HO2ME can be fitted with a control panel to monitor the device operating conditions such as: temperature and humidity inside the chamber, therapy duration. To complete the device, our company also offers a single patient Kit made of three comfortable cushions (a calf rest, an ankle rest and a heel rest), a leg guard with elastic ending and closing strap, and a medical Oxygen connection hose.
The proper oxygen flow from the HO2ME device to the limb of the patient is ensured through a POM articulated hose, while the normobaric Chamber is connected to the oxygen source through a special fast coupling external medical tube.
The oxygen source can be either a Medical Oxygen cylinder provided with a humidifier, if the treatment is performed at home, or the oxygen generating system of the Hospital. A safety valve prevents any overpressure and a suitable connection sleeve in canvas is applied to avoid any excess oxygen leakage from the normobaric Chamber. HO2ME can be fitted with a control panel to monitor the device operating conditions such as: temperature and humidity inside the chamber, therapy duration. To complete the device, our company also offers a single patient Kit made of three comfortable cushions (a calf rest, an ankle rest and a heel rest), a leg guard with elastic ending and closing strap, and a medical Oxygen connection hose.

HO2ME device is easy to use, light and safe. It can be used in a simple hospital bed or even at home if required due to the disability of the patient, as it does not need continuous assistance and maintenance by specialized staff. To make it easier and safer to transport, HO2ME is provided with a suitable semi-rigid bag fitted with handles and strap. HO2ME offers the safety and efficacy of a specialized treatment at reduced costs and increased comfort for the patient.
CERTIFICATIONS: HO2ME is a class II EC Medical Device (93/42 EEC Directive), certified by SGS and registered in the Medical Devices Directory of the National Health Service.
BENEFITS:
Device for the treatment of:
- DIABETIC, ARTERIOPATHIC, PHLEBOSTATIC AND MIXED ULCERATIONS;
- BEDSORES;
- COMPLEX WOUNDS WITH BACTERIAL AND FUNGAL INFECTIONS;
- SOFT TISSUE LESIONS CAUSED BY CORTISONE TREATMENT IN PATIENTS SUFFERING FROM CHRONIC DISABLING DISEASES;
- SKIN GRAFTS WITH ENGRAFTMENT RISKS DUE TO UNFAVOURABLE LOCAL OR GENERAL CONDITIONS;
- INFECTED BURNS AND BONE LESIONS OR OPEN BONE FRACTURES;
- TREATMENT OF MILD AND SERIOUS VENOUS INSUFFICIENCY, AS PREVENTIVE TREATMENT FOR VARICOSE VEINS AND ULCERS.
DURATION OF THE TREATMENT:
The treatment duration varies depending on the disease and on the severity of the lesion and should be established by a physician. There are no contraindications for use.
ADVANTAGES AND STRENGTH POINTS
- SGS CERTIFIED
safety
efficacy of specialised therapy
no contraindications - SMALL SIZE:
easy to transport
comfortable for the patient
low costs - RESOURCE SAVING IN TERMS OF:
time of use
time necessary for assistance and maintenance by medical staff
medication - USE:
in a simple hospital bed
at home
SPECIFICATIONS
| Product size | 600 x 350 x 350 mm |
| Construction material |
Injection polycarbonate Thickness 2.5 - 3.5 mm |
| Control panel | Thermometer, hygrometer, timer and clock |
| Weight | 3,5 kg |
| Maximum O2 Pressure | 5 torr |
| O2 Connection (outside the chamber) | Medical grade tube, 6 mm fast coupling, manufactured without latex |
| O2 Connection (inside the chamber) | POM articulated hose |
| Certification | SGS CERTIFICATE - EC 0120 |
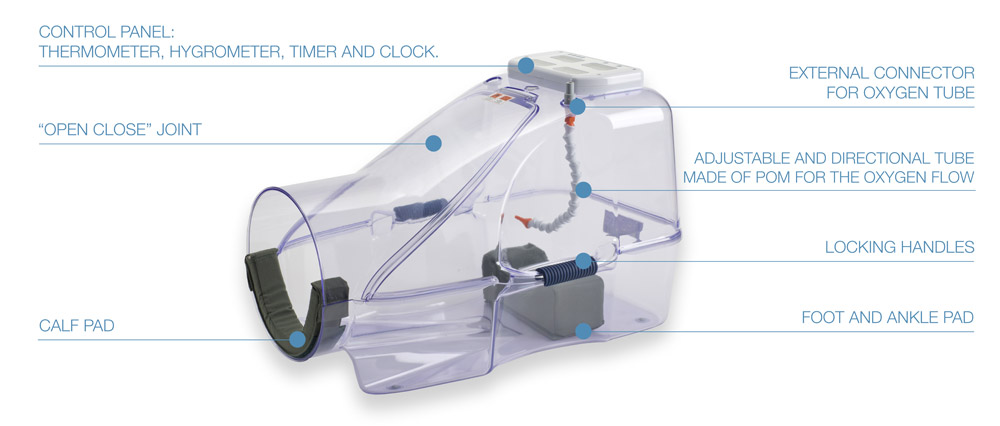
HO2ME CONSISTS OF:
- Normobaric chamber
- POM articulated hose inside the chamber
- External medical tube for connection to oxygen source
- Foot and ankle rest
- Calf rest
- 100% polyester canvas connection sleeve
- Control panel
- Semi-rigid transport bag

